Arsenic is a metalloid and belongs to the 15th group in the periodic table, having 74.92159u atomic mass. Let us explore some of the facts about Arsenic.
Arsenic has an electronegativity of 2.18, similar to phosphorous, and it can readily form covalent bonds with non-metals. Arsenic can exist in three allotropic forms, namely grey, yellow and black, of which grey allotrope is most widely used.
Arsenic is mostly in combination with sulfur and metals and appears to be crystalline. This article will explore arsenic ionization energy and other elements’ properties in detail.
Which element has a higher electronegativity than Arsenic
- Arsenic comes under the 15th group, and its electronic configuration is [Ar]3d104s24p3.
- Arsenic stands in the 3rd position in the 15th group, so the first two atoms, Nitrogen and Phosphorous, can have high electronegativities 3.04,2.19 compared to Arsenic.
Arsenic and sulfur electronegativity
Sulfur has less electronegativity than Arsenic’s electronegativity. It can be explained as follows;
| Electronegativity of Arsenic | Electronegativity of sulfur | Reasons |
|---|---|---|
| 2.18 | 2.58 | Electronegativity decreases as we go down from periods due to the increased distance from the nucleus, making less probability of attracting its valency electrons towards the nucleus. As Arsenic is present down the S atom, its electronegativity is less than As. |
Arsenic and chlorine electronegativity
Chlorine possesses more electronegativity than Arsenic. It can be shown below;
| Electronegativity of Arsenic | Electronegativity of chlorine | Reasons |
|---|---|---|
| 2.18 | 3.16 | Chlorine atom having the configuration of 1S2 2S2 2P6 3S2 3P5 requires only one electron to get the octet rule configuration for attaining stability. So, the chlorine atom can pull the electrons towards it. Hence chlorine atoms have electronegativity of 3.16 on Pauling’s scale. |
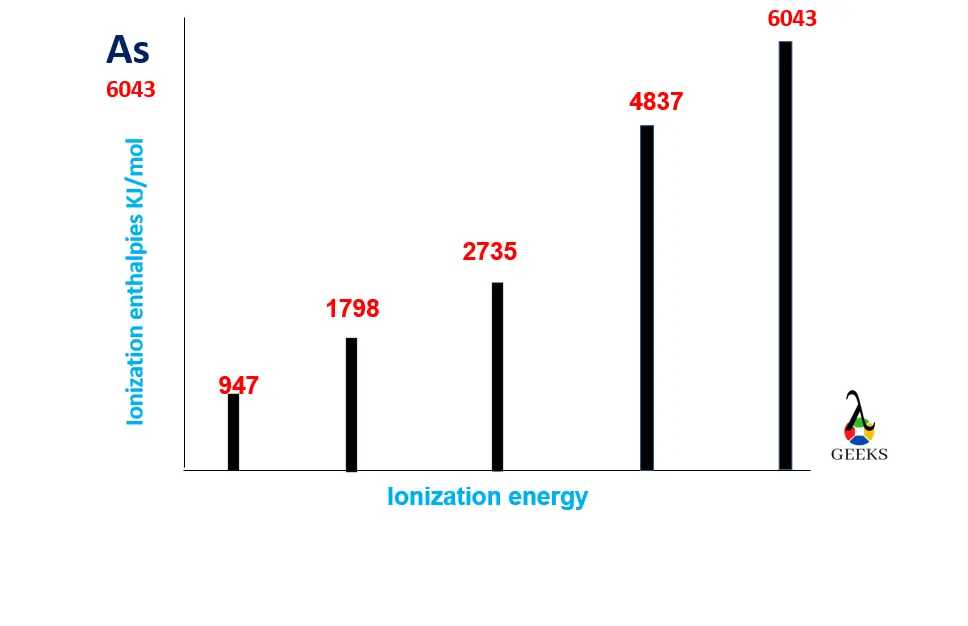
Arsenic ionization energy
Arsenic can show upto 6th ionization by removing all the outermost valency electrons from their respective orbitals since its electronic configuration is [Ar]3d104s24p3. Let us discuss its ionization energies below;
- 1st ionization energy-The first and foremost ionization energy for As is 947KJ/mol and occurs from the outermost 4P orbital.
- 2nd ionization energy-The 2nd ionization energy for As is 1798KJ/mol which is very high to remove the second electron from the 4p orbital of As.
- 3rd ionization energy-The 3rd ionization energy for As is 2735 KJ/mol, which is required to remove the 3rd electron from the 4P orbital of As.
- 4th ionization energy-The 4th ionization energy for As is 4837 KJ/mol. This high energy is needed to remove an electron from the next outermost orbital, 4S of As.
- 5th ionization energy- The 5th ionization energy for As is 6043 KJ/mol, which is high enough to remove one more electron from the 2S orbital of As.
Arsenic ionization energy graph
The arsenic ionization energy graph is shown below;
Arsenic and phosphorus ionization energy
Arsenic and Phosphorous both come under group 15. Out of these two, Phosphorous comes first. So both ionization energies differ upto some extent.
| Ionization | Ionization energy of As | Ionization energy of P | Reasons |
|---|---|---|---|
| 1st | 947 KJ/mol | 1011 KJ/mol | In both As and P, 1st electron is removed from the p-orbital. |
| 2nd | 1798 KJ/mol | 1907 KJ/mol | In As and P, the 2nd electron is also removed from the p-orbital. |
| 3rd | 2735 KJ/mol | 2914 KJ/mol | In both As and P, 3rd electron is removed from the p-orbital. |
| 4th | 4837 KJ/mol | 4963.6 KJ/mol | In As and P, the 4th electron is removed from the s-orbital. |
| 5th | 6043 KJ/mol | 6273 KJ/mol | In both As and P, the 5th electron is removed from the s-orbital, which has attained noble gas configuration. |
Arsenic and nitrogen ionization energy
Arsenic and nitrogen belong to the same group (group 15). N comes first, then after Ar, so their ionization energies differ somewhat.
| Ionization | Ionization energy of As | Ionization energy of N | Reasons |
|---|---|---|---|
| 1st | 947KJ/mol | 1402.3 KJ/mol | In both As and N, 1st electron is removed from the p-orbital. |
| 2nd | 1798KJ/mol | 2856 KJ/mol | In As and N, the 2nd electron is also removed from the p-orbital. |
| 3rd | 2735 KJ/mol | 4578.1 KJ/mol | In both As and N, 3rd electron is removed from the p-orbital. |
| 4th | 4837 KJ/mol | 7475.0 KJ/mol | In As and N, the 4th electron is removed from the s-orbital |
| 5th | 6043 KJ/mol | 944.9 KJ/mol | In both As and N, the 5th electron is removed from the s-orbital, which has attained noble gas configuration. |
Arsenic and bromine ionization energy
Bromine is the element of group 17. Hence so its ionization energy values will be different from that of As.
| Ionization | Ionization energy of As | Ionization energy of Br | Reason |
|---|---|---|---|
| 1st | 947 KJ/mol | 1139.9 KJ/mol | In both As and Br, 1st electron is removed from the p-orbital. |
| 2nd | 1798 KJ/mol | 2103 KJ/mol | In both As and Br, the 2nd electron is also removed from the p-orbital. |
| 3rd | 2735 KJ/mol | 3470 KJ/mol | In both As and Br,the 3rd electron is removed from the p-orbital. |
| 4th | 4837 KJ/mol | 4560 KJ/mol | In As and Br, the 4th electron is removed from the s-orbital. |
| 5th | 6043 KJ/mol | 5760 KJ/mol | In both As and Br, the 5th electron is removed from the s-orbital, which has attained noble gas configuration. |
Arsenic and selenium ionization energy
Selenium belongs to the 16th group, so its ionization energies differ from Arsenic.
| Ionization | Ionization energy of As | Ionization energy of Se | Reasons |
|---|---|---|---|
| 1st | 947 KJ/mol | 941.0 KJ/mol | In both Ar and Se, 1st electron is removed from the p-orbital. |
| 2nd | 1798 KJ/mol | 2045 KJ/mol | In both Ar and Se, the 2nd electron is also removed from the p-orbital. |
| 3rd | 2735 KJ/mol | 2973.7 KJ/mol | In both Ar and Se, 3rd electron is removed from the p-orbital. |
| 4th | 4837 KJ/mol | 4144 KJ/mol | The 4th electron is removed from the s-orbital and P-orbitals of Ar and Se. |
| 5th | 6043 KJ/mol | 6590 KJ/mol | In Ar and Se, the 5th electron is removed from the s-orbital and p-orbital. |
Conclusion
Arsenic is found in small concentrations on the earth’s surface. It is used in manufacturing glass. Semiconductors like gallium arsenide can convert electric current to laser light and be used as an insecticide. Lead components in car batteries get strengthened in the presence of a small amount of As.
Read more about Energy & Electronegativity:

Hi…I am Surya Satya Eluri. I have done my M.Sc in Organic Chemistry. I am very enthusiastic about the high-energy chemistry field. I love to write complicated chemistry concepts in understandable and simple words.
Let’s connect through LinkedIn: