Aluminum having the chemical formula Al, is a silvery white metal. Let us explore its electronegativity and ionization energy properties in detail.
Aluminum belongs to group 13, 3rd period, and p-block of the periodic table. The metal is soft, malleable, and lightweight. It is mostly obtained in nature as bauxite and cryolite minerals.
All the crucial features of aluminum’s electronegativity and ionization energy will be clear as we move down.
Aluminum and chlorine electronegativity
A comparison of the electronegativity values of aluminum and chlorine in Pauling units is given below.
| Electronegativity of aluminum | Electronegativity of chlorine | Explanation |
|---|---|---|
| 1.61 | 3.16 | Chlorine is more electronegative than aluminum, as chlorine is on the right-hand side of aluminum in the same period. Electronegativity increases across a period in the periodic table. |
Aluminum and fluorine electronegativity
A comparison of the electronegativity values of aluminum and fluorine in Pauling units is given below.
| Electronegativity of aluminum | Electronegativity of fluorine | Explanation |
|---|---|---|
| 1.61 | 3.98 | Fluorine is more electronegative than aluminum as non-metals are more electronegative than metals. Aluminum is a metal, whereas fluorine is a non-metal. |
Aluminum ionization energy
The atomic number of aluminum is 13 and the electronic configuration is [Ne]3s23p1.
- The first ionization energy of aluminum is 577.54 kJ/mole which is to be provided when the first electron is removed from the 3p orbital.
- The second ionization energy of aluminum is 1816.68 kJ/mole which is to be provided when the second electron is removed from the 3s orbital.
- The third ionization energy of aluminum is 2744.78 kJ/mole which is to be provided when the third electron is removed from the 3s orbital.
- The fourth ionization energy of aluminum is 11577.50 kJ/mole which is to be provided when the fourth electron is removed from the 2p orbital.
Aluminum ionization energy graph
The ionization energy graph of aluminum is drawn by taking ionization energy (in kJ/mole unit) in the y-axis and the ionization number in the x-axis.
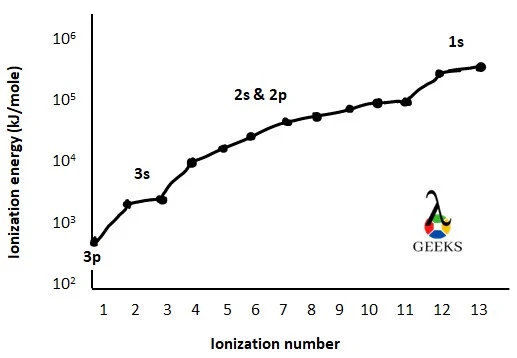
Aluminum and boron ionization energy
Both aluminum and boron belong to the same group but in different periods. Aluminum belongs to the third period whereas boron is to the second period. A comparison of their ionization energy is mentioned below.
| First ionization energy of aluminum | First ionization energy of boron | Explanation |
|---|---|---|
| 577.54 kJ/mole | 800.64 kJ/mole | The first ionization energy of boron is higher than that of aluminum as in the periodic table, down the group, ionization energy decreases. Aluminum which is just below boron in the same group (group 13), has a higher ionization energy value. |
Aluminum and magnesium ionization energy
Both aluminum and magnesium belong to the same period but in a different groups. Aluminum belongs to group 13 whereas magnesium is to group 2. A comparison of their ionization energy is mentioned below.
| First ionization energy of aluminum | First ionization energy of magnesim | Explanation |
|---|---|---|
| 577.54 kJ/mole | 737.75 kJ/mole | The electronic configurations of aluminum and magnesium are [Ne]3s23p1 and [Ne]3s2 respectively. The ionization energy of magnesium is higher than that of aluminum as the removal of an outermost electron from a stable, fully filled 3s orbital requires more energy than the removal of the same from a partially filled 3p orbital. |
Aluminum and chromium ionization energy
The electronic configurations of aluminum and chromium are [Ne]3s23p1 and [Ar]3d54s1 respectively. A comparison of their ionization energy is mentioned below.
| First ionization energy of aluminum | First ionization energy of chromium | Explanation |
|---|---|---|
| 577.54 kJ/mole | 652.87 kJ/mole | The difference between ionization energy values between aluminum and chromium is 75.33 kJ/mole. Chromium has a higher value of first ionization than aluminum because of the high attraction between the outermost electron (4s electron) and the nucleus in the case of chromium compared to aluminum. |
Conclusion

Hello, I am Tuluma Das, Completed my Ph.D. in Organic Chemistry from the Indian Association for the Cultivation of Science. I have a total of 9 years of research experience including a Ph.D. and Postdoc and 3 years of teaching experience. I have published 7 papers so far in international journals. Let’s connect through Linkedin :