Aluminium bromide is a halogenated salt which is a hygroscopic molecule. Let us explore Aluminium bromide in detail.
Aluminium bromide is formed upon the reaction of hydrobromic acid and elemental Aluminium metal. It is also prepared for direct bromination of aluminium metal. Due to electronic deficiency at the aluminium center, it exists as a dimeric form, where 2 aluminium are connected via 3C-4e bonding.
The monomer has shape trigonal planar geometry where the Br-Al-Br bond angle is near about 1200 and central Aluminium sp2 hybridized. Let us see some basic properties of Aluminium bromide like melting or boiling point, oxidation state, reaction tendency, density, and viscosity in the following part of the article.
1. Aluminium bromide IUPAC name
IUPAC (International Union of Pure and Applied Chemistry) gives the name for AlBr3 as aluminium tribromide or aluminium(III) bromide.
2. Aluminium bromide chemical formula
AlBr3 is the chemical formulation of monomeric Aluminium bromide, but the dimeric form is Al2Cl6 where Aluminium is known as Al and bromine is Br.
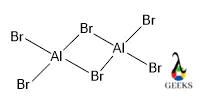
Aluminium Bromide
3. Aluminium bromide CAS number
Aluminium bromide has two different CAS numbers (up to the ten-digit numeric value given by Chemical Abstracts Service),
- 7727-15-3 (for anhydrous monomeric)
- 7784-11-4 (for hexahydrated form)
4. Aluminium bromide Chem Spider ID
22818 is the Chem Spider ID (given by the Royal Society of Chemistry) for Aluminium bromide.
5. Aluminium bromide chemical classification
Aluminium bromide can be classified into the following categories-
- Aluminium bromide is an inorganic halogenated salt
- Aluminium bromide is a hygroscopic molecule
- Aluminium bromide is a strong electrolyte
- Aluminium bromide is a good lewis acid
- Aluminium bromide is a good catalyst
- Aluminium bromide is a reference electrode
6. Aluminium bromide molar mass
Aluminium bromide has 266.69 g/mol as molar mass whereas the atomic mass of aluminium 26.98 and the atomic mass of 3 bromine are 79.04*3= 237.12.
7. Aluminium bromide color
Pure Aluminium bromide is colorless but impure molecule has color yellowish or even red-brownish due to being iron-containing.
8. Aluminium bromide viscosity
Solid Aluminium bromide has 0 viscosity as the property is only for fluid exerting the frictional force and the solid form is highly water soluble.
9. Aluminium bromide molar density
The molar density of the solid monomeric Aluminium bromide is 3.32 g/cm3.
10. Aluminium bromide melting point
The melting point for anhydrous Aluminium bromide is 97.50 or 370.5K and for hexahydrate form, it is 930C or 366K.
11. Aluminium bromide boiling point
Only the anhydrous form of Aluminium bromide has boiling point which is 2550C or 528K.
12. Aluminium bromide state at room temperature
Aluminium bromide exists in solid crystalline form at room temperature.
13. Aluminium bromide ionic bond
The bond between Aluminium and bromine is ionic because Aluminium ions easily polarized larger bromide ions due to their higher ionic potential as per Fajan’s rule. So, there will be a strong electrostatic force present between Aluminium and bromine which is ionic interaction.
14. Aluminium bromide ionic radius
The ionic radius of Aluminium and bromine are 184 pm and 185 pm respectively as they formed ion ionic bonds.
15. Aluminium bromide electron configurations
Electronic configurations are the arrangement of electrons by their number and position. Let us discuss the electronic configuration for AlBr3.
- The electronic configuration of Aluminium is [Ne]3s23p1
- The electronic configuration of Bromine is [Ar] 3d104s24p5
- The electronic configuration of a molecule or complex is unpredictable because its only for elements.
16. Aluminium bromide oxidation state
The oxidation state of the Aluminium in Aluminium bromide is +3 because it exists as Al3+ and the oxidation state of the bromine is -1 because it exists as bromide or Br–.
17. Aluminium bromide acidity/alkaline
Aluminium bromide has an acidic character rather it is lewis acid, and it increases due to three electronegative bromine atoms pulling the electron density towards themselves and the Aluminium center becomes more positive and can accept more electron density.
18. Is Aluminium bromide odorless?
Aluminium bromide has a characteristic pungent smell like ammonia.
19. Is Aluminium bromide paramagnetic?
Paramagnetism is a property depending on the availability of the number of unpaired electrons. Let us see whether Aluminium bromide is paramagnetic or not.
Monomeric Aluminium bromide is paramagnetic due to 3 unpaired electrons over Aluminium but the dimeric form is diamagnetic due to the pair of all electrons. The magnetic susceptibility value of Aluminium bromide is −21*10−6 cm3/mol.
20. Aluminium bromide hydrates
Aluminium bromide is a hexahydrate molecule which means per Aluminium bromide contains 6 water molecules in its crystal which can represent AlBr3.6H2O.
21. Aluminium bromide crystal structure
The anhydrous form of Aluminium bromide has a monoclinic crystal structure in its lattice form, where the lattice constant, a = 0.7512 nm, b = 0.7091 nm, c =1.0289 nm, and α = 900, β = 96.440, and γ = 900. Four
22. Aluminium bromide polarity and conductivity
Aluminium bromide is non-polar but conductive in nature and the supporting reasons are,
- The molecule can be ionized in the Al3+ and Br–. Both ions are highly conductive and have higher mobility due to higher charge density.
- Aluminium bromide is rapidly soluble in water to get dissociated into ions.
- The dipole moment of AlBr3 flows from Al to Br.
- Due to the trigonal planar geometry, which is a symmetric molecule, the resultant dipole moment will be 0 making the molecule non-polar AlBr3 as well.
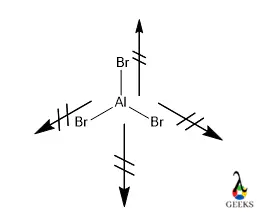
aluminium bromide
23. Aluminium bromide reaction with acid
Being a lewis acid Aluminium bromide does not react with any acidic molecule but sometimes it reacts with a strong inorganic mineral acid like sulfuric acid.
H2SO4 + AlBr3 = HBrO3 + AlSO4
24. Aluminium bromide reaction with base
Aluminium bromide can react with some lewis bases as it is strong lewis acid and form an adduct with that base. The electron deficiency is the main driving force for reacting Aluminium bromide with a suitable lewis base.
NH3 + AlBr3 = H3N-AlBr3
25. Aluminium bromide reaction with oxide
Aluminium bromide can react with superoxide and replace the Aluminium and form Aluminium oxide. Superoxide should be of some metal having higher electropositivity than Aluminium.
KO2 + AlBr3 = KBr + Al2O3
26. Aluminium bromide reaction with the metal
Aluminium bromide can react with Aluminium to form a dimeric form of the molecule or it can react with a complex of a transition metal having higher reduction potential.
AlBr3 + COCl2 → COBr2 + AlCl2Br
Conclusion
Aluminium bromide can be used as a catalyst for the Friedel-craft where the alkyl group should be incorporated in any aromatic ring. It is also used for any epoxide ring opening reaction promoted by lewis acid. Aluminium bromide can be disproportionate at room temperature so it always tries to exit in the dimeric form.
Read more following properties

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.