| Potential Energy Type | Definition |
|---|---|
| Gravitational Potential Energy | Energy stored in an object due to its height above the ground |
| Elastic Potential Energy | Energy stored in a stretched or compressed object, like a spring |
| Chemical Potential Energy | Energy stored in the bonds of atoms and molecules |
| Nuclear Potential Energy | Energy stored in the nucleus of an atom |
| Electric Potential Energy | Energy stored in an electric field due to the position of charged particles |
What is Potential Energy?
Potential energy is a fundamental concept in physics that refers to the energy an object possesses due to its position or configuration. It is a form of stored energy that an object has the potential to convert into other forms of energy, such as kinetic energy or thermal energy. In simpler terms, potential energy is the energy an object has when it is at rest or in a stable state.Definition of Potential Energy
Potential energy can be defined as the energy an object possesses due to its position or state. It is the energy that is stored within an object and can be released or converted into other forms of energy when certain conditions are met. There are various types of potential energy, including gravitational potential energy, elastic potential energy, chemical potential energy, nuclear potential energy, and more.Explanation of Potential Energy as Stored Energy
To understand potential energy as stored energy, let’s consider a few examples.- Gravitational Potential Energy: When an object is lifted to a certain height above the ground, it gains gravitational potential energy. This energy is stored in the object and can be converted into kinetic energy when the object is released and falls towards the ground.
- Elastic Potential Energy: When a spring is stretched or compressed, it gains elastic potential energy. This energy is stored in the spring and can be converted into kinetic energy when the spring is released and returns to its original shape.
- Chemical Potential Energy: Chemical substances, such as fuels or batteries, contain chemical potential energy. This energy is stored in the bonds between atoms and can be released through chemical reactions, producing other forms of energy like heat or electrical energy.
- Nuclear Potential Energy: Atomic nuclei contain nuclear potential energy. This energy is stored in the strong forces that hold the nucleus together. It can be released through nuclear reactions, such as nuclear fission or fusion, where a small amount of mass is converted into a large amount of energy.
Potential Energy Unit and Measurement
The unit of potential energy depends on the type of potential energy being considered. Here are some common units of measurement for different types of potential energy:- Gravitational Potential Energy: The unit of measurement for gravitational potential energy is the joule (J). It can be calculated using the formula:
![]()
- Elastic Potential Energy: The unit of measurement for elastic potential energy is also the joule (J). It can be calculated using the formula:
![]()
- Chemical Potential Energy: The unit of measurement for chemical potential energy is also the joule (J). It can be calculated based on the specific chemical reaction and the energy released or absorbed during the reaction.
- Nuclear Potential Energy: The unit of measurement for nuclear potential energy is also the joule (J). It can be calculated based on the mass defect and the conversion of mass into energy according to Einstein’s famous equation, (E = mc^2).
Types of Potential Energy
Gravitational Potential Energy
Gravitational potential energy is the energy stored in an object due to its position in a gravitational field. It is the energy an object possesses by virtue of its height above the ground or any reference point. The formula to calculate gravitational potential energy is given by: ![]()
![]()
Elastic or Spring Potential Energy
Elastic potential energy is the energy stored in an object when it is deformed or stretched. It is commonly associated with objects like springs or rubber bands. The amount of elastic potential energy stored in an object depends on its stiffness and the amount of deformation. The formula to calculate elastic potential energy is given by: ![]()
![]()
Chemical Potential Energy
Chemical potential energy is the energy stored in chemical bonds between atoms and molecules. It is released or absorbed during chemical reactions. The amount of chemical potential energy stored in a substance depends on the types and arrangements of atoms in its molecules. For example, fossil fuels like gasoline contain chemical potential energy that is released when burned. Similarly, food contains chemical potential energy that our bodies convert into usable energy.Electric Potential Energy
Electric potential energy is the energy stored in an object due to its position in an electric field. It is associated with the interaction between electric charges. The formula to calculate electric potential energy is given by: ![]()
![]()
Nuclear Potential Energy
Nuclear potential energy is the energy stored in the nucleus of an atom. It is associated with the strong nuclear force that holds the protons and neutrons together. Nuclear potential energy can be released or absorbed during nuclear reactions, such as nuclear fission or fusion. The amount of nuclear potential energy stored in an atom depends on the binding energy of its nucleus.Potential Energy and Forces

The role of gravitational force in potential energy
Gravitational force plays a crucial role in potential energy. When an object is lifted to a certain height above the ground, it gains gravitational potential energy. This energy is a result of the gravitational force exerted on the object due to its position in a gravitational field. The higher the object is lifted, the greater its potential energy becomes. The formula to calculate gravitational potential energy is given by: ![]()
![]()
The role of electrostatic force in potential energy
Electrostatic force is another force that contributes to potential energy. It is the force of attraction or repulsion between charged particles. When work is done to move charged particles against the electrostatic force, their potential energy increases. The formula to calculate electrostatic potential energy is given by: ![]()
![]()
Potential energy as a conservative force
Potential energy is often associated with conservative forces. A conservative force is a force that does work on an object, but the total mechanical energy of the object remains constant. In other words, the work done by a conservative force can be fully recovered as potential energy. Some examples of conservative forces include gravitational force, elastic force, and electrostatic force. These forces store potential energy in the system, which can be released or converted into other forms of energy. For example, consider a spring that is compressed by a distance (x). The potential energy stored in the spring, known as elastic potential energy, can be calculated using the formula: ![]()
![]()
Potential Energy and Kinetic Energy
Potential energy and kinetic energy are two forms of energy that objects can possess. They are both related to the motion and position of an object. Let’s explore the relationship between potential and kinetic energy, how potential energy can be converted into kinetic energy, and scenarios where potential energy is high and kinetic energy is low.The Relationship Between Potential and Kinetic Energy
Potential energy is the energy stored in an object due to its position or configuration. It is the energy an object possesses because of its potential to do work. There are different types of potential energy, such as gravitational potential energy, elastic potential energy, chemical potential energy, and nuclear potential energy. Gravitational potential energy is the energy an object possesses due to its height above the ground. It can be calculated using the formula: ![]()
![]()
![]()
Conversion of Potential Energy to Kinetic Energy
The conversion of potential energy to kinetic energy occurs when an object moves from a higher position to a lower position. This can happen due to the force of gravity or the release of stored energy in a spring. Let’s take an example of a ball being dropped from a height. Imagine you are standing on a tall building and you drop a ball from the rooftop. Initially, the ball has a high potential energy due to its height above the ground. As the ball falls, its potential energy decreases while its kinetic energy increases. At the moment the ball hits the ground, all of its potential energy is converted into kinetic energy. This is because the ball has reached its lowest position and has no more potential energy left. The conversion of potential energy to kinetic energy can also be observed in a simple pendulum. When a pendulum is at its highest point, it has maximum potential energy. As it swings down, its potential energy decreases while its kinetic energy increases. At the lowest point of the swing, all of the potential energy is converted into kinetic energy.Scenarios When Potential Energy is High and Kinetic Energy is Low
There are several scenarios where an object may have high potential energy and low kinetic energy. Let’s consider a few examples:- A book placed on a table: In this scenario, the book has potential energy due to its position above the ground. However, since it is at rest, its kinetic energy is low.
- A compressed spring: When a spring is compressed, it stores potential energy in the form of elastic potential energy. The spring has the potential to release this energy and convert it into kinetic energy when it is released.
- A roller coaster at the top of a hill: At the highest point of a roller coaster ride, the cars have high potential energy due to their height above the ground. However, their kinetic energy is low because they are momentarily at rest before gravity pulls them down the hill.
Interesting Aspects of Potential Energy
Potential energy is a fascinating concept in the field of physics that describes the energy stored in an object due to its position, shape, or composition. It is a fundamental concept that helps us understand various phenomena in the natural world. In this article, we will explore some interesting aspects of potential energy and delve into its different forms and properties.Why potential energy can be negative
One intriguing aspect of potential energy is that it can be negative. This occurs when the reference point for potential energy is chosen in such a way that the object’s actual energy is lower than the reference point. For example, in the case of gravitational potential energy, if we choose the reference point to be at a higher elevation than the object, then the potential energy of the object at a lower elevation will be negative. This negative potential energy signifies that the object has less energy than when it is at the reference point.Why potential energy is zero at infinity
Another interesting aspect of potential energy is that it becomes zero when an object is infinitely far away from the source of the potential. This is particularly evident in the case of gravitational potential energy. As an object moves farther away from a massive body, such as the Earth, the gravitational potential energy decreases. At an infinite distance, the potential energy becomes zero. This implies that the object has no potential energy when it is infinitely far away from the gravitational field.Why potential energy is maximum at extreme position
In systems where potential energy is associated with a restoring force, such as in the case of elastic potential energy, the potential energy is maximum at extreme positions. For example, consider a spring that is stretched or compressed. At the extreme positions, where the spring is either fully stretched or fully compressed, the potential energy is at its maximum. This occurs because the restoring force of the spring is at its maximum at these points, storing the maximum amount of potential energy.Potential energy during phase changes
During phase changes, such as the transition from a solid to a liquid or a liquid to a gas, potential energy plays a crucial role. The potential energy of the particles in a substance changes as they undergo phase transitions. For example, during the melting of ice, the potential energy of the water molecules increases as they break free from the rigid structure of the ice crystal. Similarly, during evaporation, the potential energy of the liquid molecules increases as they escape from the attractive forces of the liquid phase. These changes in potential energy are essential for understanding the behavior of substances during phase transitions.Real-life Examples of Potential Energy
Potential Energy in Everyday Life
In our daily lives, we encounter potential energy in numerous situations. One common example is a roller coaster at the top of a hill. As the roller coaster ascends to the highest point, it gains gravitational potential energy. This energy is a result of its position relative to the ground. When the roller coaster is released, the potential energy is converted into kinetic energy, resulting in an exhilarating ride. Another example of potential energy in everyday life is a stretched rubber band. When a rubber band is stretched, it gains elastic potential energy. This energy is stored in the stretched bonds between the rubber molecules. When the rubber band is released, the potential energy is transformed into kinetic energy, causing the rubber band to snap back to its original shape.Potential Energy in Physics and Engineering
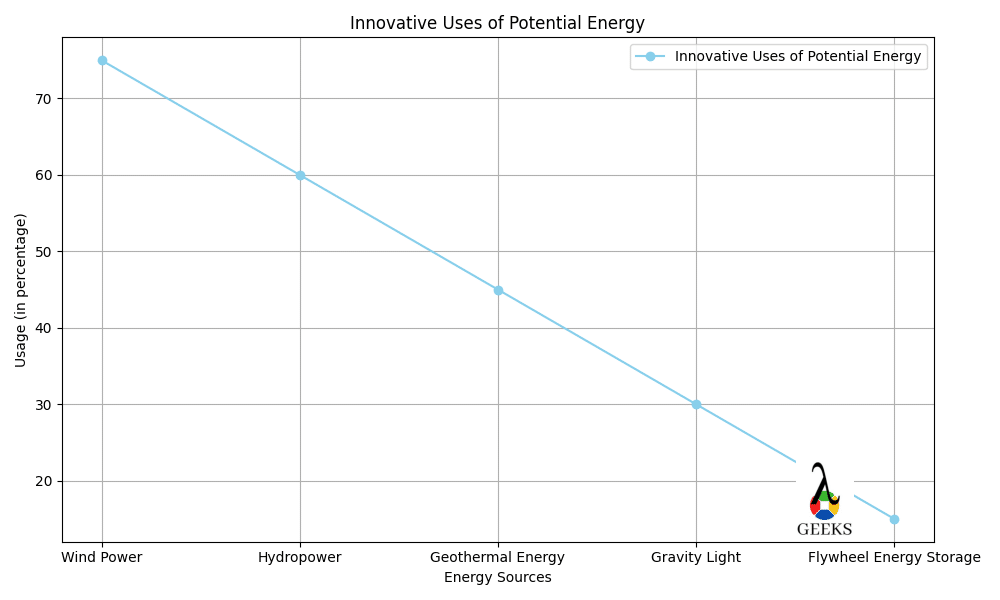
In the field of physics and engineering, potential energy plays a crucial role in understanding and designing various systems. One significant example is the potential energy associated with chemical bonds. When atoms combine to form molecules, they create chemical potential energy. This energy is released or absorbed during chemical reactions, such as burning wood or digesting food. Nuclear potential energy is another example that is harnessed in nuclear power plants. The energy stored within atomic nuclei is released through nuclear reactions, such as nuclear fission or fusion. This energy is then converted into electricity, providing power to homes and industries.Innovative Uses of Potential Energy
References
Citing sources of information used in the blog post.
In writing this blog post, I have gathered information from various reliable sources to ensure accuracy and credibility. Here are the references I used:- Potential Energy – I referred to the textbook “Physics: Principles with Applications” by Douglas C. Giancoli for a comprehensive understanding of potential energy. This book provided detailed explanations and examples of different types of potential energy, such as gravitational potential energy, elastic potential energy, chemical potential energy, and nuclear potential energy.
- Mechanical Potential Energy – To explain mechanical potential energy and its relation to stored energy in an object, I relied on the book “Conceptual Physics” by Paul G. Hewitt. This book provided clear explanations and real-life examples to illustrate the concept of energy of position and energy of configuration.
- Gravitational Potential Energy – I gathered information on gravitational potential energy from the website of the Physics Classroom. This online resource provided detailed explanations and examples of how the energy of an object at rest can be attributed to its height and the force of gravity acting on it.
- Elastic Potential Energy – To explain elastic potential energy and its connection to deformation, I consulted the book “University Physics” by Hugh D. Young and Roger A. Freedman. This book provided a comprehensive explanation of how energy can be stored in elastic materials and released when they return to their original shape.
- Chemical Potential Energy – I referred to the book “Chemistry: The Central Science” by Theodore L. Brown, H. Eugene LeMay, and Bruce E. Bursten to understand the concept of chemical potential energy. This book provided insights into how energy is stored in chemical bonds and released during chemical reactions.
- Nuclear Potential Energy – To explain nuclear potential energy and its relation to atomic nuclei, I relied on the book “Modern Physics” by Kenneth S. Krane. This book provided a detailed explanation of how energy is stored in atomic nuclei and released during nuclear reactions.
- Energy Due to Motion – I gathered information on the energy due to motion from the website of the Khan Academy. This online resource provided clear explanations and examples of how the energy of an object can be attributed to its motion, velocity, and mass.
- Energy Due to Gravity – To explain the energy due to gravity, I referred to the book “Fundamentals of Physics” by David Halliday, Robert Resnick, and Jearl Walker. This book provided a comprehensive explanation of how the energy of an object can be attributed to its position in a gravitational field.
- Energy Due to Elasticity – I gathered information on the energy due to elasticity from the website of the HyperPhysics project. This online resource provided detailed explanations and examples of how the energy of an object can be stored in elastic materials and released when they are deformed.
- Energy Due to Forces – To explain the energy due to forces, I relied on the book “Physics for Scientists and Engineers” by Raymond A. Serway and John W. Jewett. This book provided a comprehensive explanation of how the energy of an object can be attributed to the work done by external forces.
Frequently Asked Questions

1. What is potential energy in physics?
Potential energy in physics refers to the energy that an object possesses due to its position or configuration. It is stored energy that can be converted into other forms, such as kinetic energy, when the object is in motion.2. Why is potential energy negative of work done?
Potential energy is negative of work done because work done is the transfer of energy from one form to another. When work is done on an object, its potential energy decreases, resulting in a negative change in potential energy.3. Where is elastic potential energy stored?
Elastic potential energy is stored in objects that can be deformed or stretched, such as a compressed spring or a stretched rubber band. The energy is stored in the object’s ability to return to its original shape or position when the deforming force is removed.4. Does potential energy increase with temperature?
In most cases, potential energy does not directly depend on temperature. However, in certain systems, such as those involving chemical reactions or phase changes, potential energy can be influenced by temperature changes.5. What potential energy does the sun have?
The sun primarily possesses nuclear potential energy. It generates energy through nuclear fusion, where hydrogen atoms combine to form helium, releasing a tremendous amount of energy in the process.6. Is potential energy a vector or scalar quantity?
Potential energy is a scalar quantity. Unlike vector quantities, such as velocity or force, potential energy does not have a specific direction associated with it. It only has magnitude.7. When potential energy is converted to kinetic energy?
Potential energy is converted to kinetic energy when an object’s position or configuration changes, causing the potential energy to decrease and the kinetic energy to increase. This typically occurs when an object falls freely under the influence of gravity.8. Why is potential energy always negative?
Potential energy can be negative because it is defined as the work required to bring an object from infinity to its current position. Since work done by attractive forces is negative, potential energy associated with such forces is also negative.9. Does potential energy change during a phase change?
During a phase change, such as the transition from solid to liquid or liquid to gas, the potential energy of the substance does not change. Instead, the energy is used to break or form intermolecular forces, resulting in a change in the substance‘s state.10. Where is potential energy stored?
Potential energy is stored in various forms, including gravitational potential energy (due to height), elastic potential energy (in deformed objects), chemical potential energy (in chemical bonds), and nuclear potential energy (in atomic nuclei).
The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.