What is the product of H2SO3 and K2CO3?
K2CO3 is known as potassium carbonate. A sulphurous acid has a chemical formula of H2SO3. Let us see the reaction between H2SO3 & K2CO3.
Potassium carbonate (K2CO3) is a white salt soluble in water it is deliquescent in nature often appearing as a damp and wet solid. Sulphureous acid is a colourless liquid which is having burning sulphur smell.
H2SO3+ K2CO3 reaction gives us the idea about the product formed in this reaction, how to balance the reaction, titration enthalpy of reaction and many more facts.
What type of reaction is H2SO3 + K2CO3?
When sulphurous acid reacts with potassium carbonate it forms carbonic acid and potassium sulphite.
H2SO3+ K2CO3 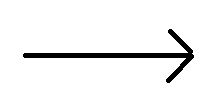 K2SO3 + H2O+ CO2
K2SO3 + H2O+ CO2
What type of reaction is H2SO3 + K2CO3?
H2SO3 + K2CO3 is a double displacement reaction.
How to balance H2SO3 + K2CO3?
The H2SO3 + K2CO3 reaction is balanced by the following steps.
- For the reactions to be balanced the number of atoms of each element present on both the reactant and product side must be equal.
- Create an equation for each element present in the reaction, representing the number of atoms of an element present in the product and reactant.
| Atom | Reactant Side | Product Side |
| Hydrogen | 2 | 2 |
| Sulphur | 1 | 1 |
| Oxygen | 6 | 6 |
| Potassium | 2 | 2 |
| Carbon | 1 | 1 |
- All the elements have the same number of atoms in both the reactant and product side hence balanced reaction is as follows.
H2SO3+ K2CO3 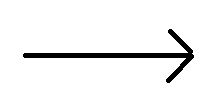 K2SO3 + H2O+ CO2
K2SO3 + H2O+ CO2
H2SO3 + K2CO3 titration
H2SO3 + K2CO3 is a volumetric titration. This titration is carried out as follows.
Apparatus and Chemicals Required
50 mL burette, pipette, conical flask, measuring flask, Beaker, funnel, distilled water, Sulphurous acid, sodium carbonate.
Indicator
Methyl red indicator is used in the titration. Which gives the equilibrium point between the acid and base.
Procedure
- Standard H2SO3 and K2CO3 solutions were prepared with the same concentration.
- Then 10ml H2SO3 is taken in a conical flaskand K2CO3 solution is filled in the burette.
- K2CO3 solution is added dropwise in a conical flask containing H2SO3 and methyl red indicator.
- Titrate till the colour changes from orange to light pink.
- When acid H2SO3 isneutralised with baseK2CO3 it gives the endpoint indicated by colourchange.
- The final reading was noted and determined the amount of K2CO3 required to neutralize the H2SO3 by the M1V1 =M2V2 formula.
H2SO3 + K2CO3 net ionic equation
The net ionic equation of H2SO3 + K2CO3 is.
- The chemical equation with the physical state of both the product and reactant sides is as follows.
- H2SO3(aq) + K2CO3 (s)
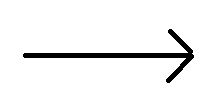 K2SO3 (S) + CO2 (g) + H2O (aq).
K2SO3 (S) + CO2 (g) + H2O (aq). - In this reaction salt of acid and base dissociates into ions. And pure solid substances do not dissociate into ions.
- 2H+ + CO2-
 CO2 + H2O
CO2 + H2O
H2SO3 + K2CO3 conjugate pairs
- H2SO3 has a conjugate pair of HSO3– .
- K2CO3 does not contain a proton and hence does not have conjugate pairs.
H2SO3 and K2CO3 intermolecular forces
- H2SO3 molecule contains strong hydrogen bonding between two H2 atoms.
- K2CO3 molecule exhibits London Dispersion forces.
H2SO3 + K2CO3 reaction enthalpy
H2SO3 + K2CO3 reaction shows negative reaction enthalpy.
Is H2SO3 + K2CO3 a buffer solution?
The H2SO3 + K2CO3 reaction does not form a buffer solution because it contains acid.
Is H2SO3 + K2CO3 a complete reaction?
H2SO3 + K2CO3 is the complete reaction becausestable products like K2SO3, CO2 & H2O are formed in this reaction.
Is H2SO3 + K2CO3 an exothermic or endothermic reaction?
H2SO3 + K2CO3 is an exothermic reaction as it has a negative enthalpy of reaction.
Is H2SO3 + K2CO3 a redox reaction?
H2SO3 + K2CO3 is not a redox reaction because oxidation and reduction do not occur in this reaction.
Is H2SO3 + K2CO3 a precipitation reaction?
H2SO3 + K2CO3 is not a precipitation reaction because the product’s precipitates are not formed.
Is H2SO3 + K2CO3 reversible or irreversible reaction?
H2SO3 + K2CO3is an irreversible reaction because the product formed cannot back converted into a reactant.
Is H2SO3 + K2CO3 displacement reaction?
H2SO3 + K2CO3 is a double displacement reaction because displacement in the molecule was observed in the reactant.
Conclusion
H2SO3 + K2CO3 is an acid-base double displacement reaction in this reaction K2SO3 salt CO2 and H2O are formed. H2SO3 + K2CO3 form stable product it does not undergo oxidation as well as reduction. It is a complete reaction.

Hi….I am Darshana Fendarkar, I have completed my Ph.D. from the University of Nagpur. My area of specialization is Inorganic Chemistry.
I have an experience as a Chemist at Earthcare Pvt. Ltd. Also I have 2 years of experience in teaching. Currently, I am working with Lambdageek as a Subject Matter Expert.