Introduction
Light, the fundamental entity that allows us to see and perceive the world around us, has always fascinated scientists with its dual nature. It exhibits both wave and particle characteristics, which seems contradictory at first. This phenomenon, known as wave-particle duality, has been a subject of intense study and debate in the field of physics. The wave nature of light explains its ability to diffract and interfere, while the particle nature is evident in phenomena like the photoelectric effect. Understanding why light behaves in this peculiar way has been a significant challenge for scientists, leading to groundbreaking discoveries and advancements in our understanding of the nature of light.
Key Takeaways
| Wave Characteristics | Particle Characteristics |
|---|---|
| Diffraction | Photoelectric effect |
| Interference | Compton scattering |
| Polarization | Photon emission |
| Refraction | Absorption |
| Reflection | Scattering |
Understanding the Nature of Light
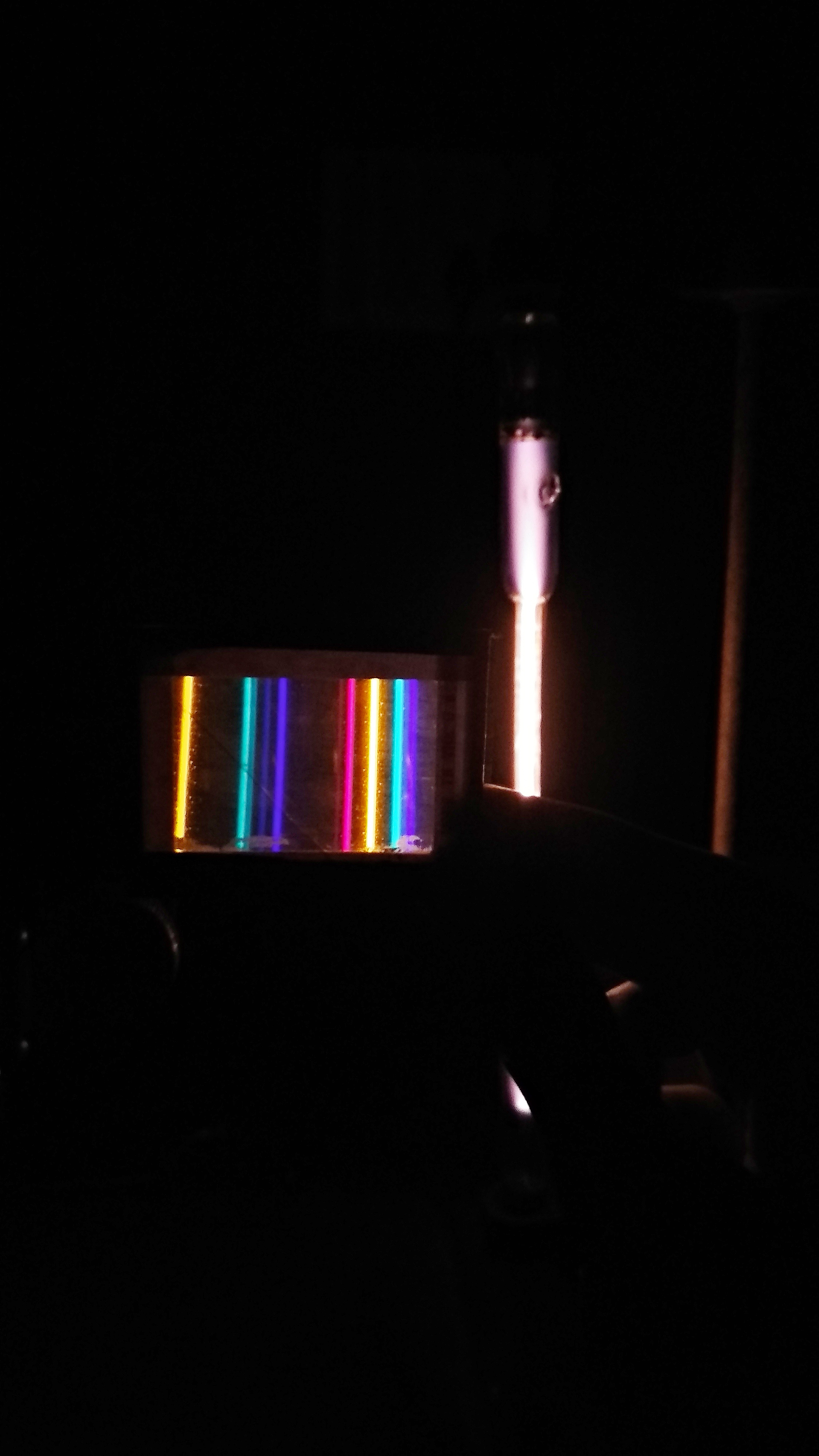
Light is a fascinating phenomenon that has captivated scientists and philosophers for centuries. It plays a crucial role in our everyday lives, allowing us to see the world around us. But what exactly is light, and how does it behave? In this article, we will delve into the nature of light, exploring its history and the concept of wave-particle duality.
Brief History of Light Theory
The study of light dates back to ancient times, with early philosophers proposing various theories to explain its nature. One of the earliest theories was the emission theory, which suggested that light is emitted from the eyes and illuminates objects. This theory was later challenged by the intromission theory, which proposed that light is emitted by objects and enters the eyes.
In the 17th century, Sir Isaac Newton conducted experiments with prisms and discovered that white light is composed of a spectrum of colors. This led to the development of the particle theory of light, which stated that light consists of tiny particles called “corpuscles.” According to this theory, the different colors of light are a result of variations in the size and speed of these particles.
However, in the early 19th century, Thomas Young’s double-slit experiment provided evidence for the wave nature of light. Young observed that when light passed through two closely spaced slits, it created an interference pattern, similar to the pattern produced by water waves. This experiment challenged the particle theory and paved the way for the wave theory of light.
The Concept of Wave-Particle Duality
The wave-particle duality of light is a fundamental concept in quantum mechanics. It suggests that light can exhibit both wave-like and particle-like behavior, depending on the experimental setup. This duality was first proposed by Albert Einstein in 1905 to explain the photoelectric effect, where light can eject electrons from a material.
According to the wave-particle duality, light can be described as both a wave and a stream of particles called photons. As a wave, light exhibits properties such as interference and diffraction, which are characteristic of wave phenomena. On the other hand, as a particle, light interacts with matter in discrete packets of energy, known as quanta.
The wave nature of light is further supported by phenomena like the diffraction of light around obstacles and the interference of light waves. These phenomena can be explained using the wave equation and the concept of superposition, where multiple waves combine to form a resultant wave.
On the other hand, the particle nature of light is evident in phenomena like the photoelectric effect and Compton scattering. The photoelectric effect occurs when light of a certain frequency ejects electrons from a material, while Compton scattering involves the interaction of photons with electrons, resulting in a change in their wavelength.
The wave-particle duality is not limited to light alone but applies to all particles in the quantum world. This duality is described by the de Broglie wavelength, which relates the momentum of a particle to its wavelength. The wavelength of a particle is inversely proportional to its momentum, indicating that particles with higher momentum have shorter wavelengths.
In conclusion, the nature of light is a complex and intriguing topic that has been studied for centuries. The wave-particle duality of light, as described by quantum mechanics, allows us to understand its behavior both as a wave and a particle. This duality has revolutionized our understanding of the microscopic world and continues to be a subject of ongoing research and exploration.
Light as a Wave

Wave Theory of Light
Light, as we perceive it, is not just a stream of particles but also exhibits wave-like behavior. This concept, known as the wave theory of light, revolutionized our understanding of electromagnetic radiation. According to this theory, light is an electromagnetic wave that propagates through space, carrying energy and information.
To better understand the wave nature of light, let’s delve into some key aspects:
Energy Quantization and the Photon
In the early 20th century, quantum mechanics emerged, revealing that energy is quantized. This means that energy exists in discrete packets, known as quanta. In the case of light, these quanta are called photons. Each photon carries a specific amount of energy, which is directly proportional to its frequency. This relationship is described by the equation:
![]()
where (E) represents the energy of a photon, (h) is Planck’s constant, and (f) denotes the frequency of the light wave.
Interference and Diffraction
One of the most fascinating phenomena that demonstrates the wave nature of light is interference. When two or more light waves overlap, they can either reinforce or cancel each other out, depending on their relative phases. This interference pattern can be observed in various experiments, such as Young’s double-slit experiment.
Diffraction is another characteristic of waves, including light waves. It refers to the bending or spreading of waves as they encounter an obstacle or pass through a narrow opening. Diffraction patterns can be observed when light passes through a small aperture or encounters an edge.
Evidence of Light’s Wave Nature
Several experiments provide evidence for the wave nature of light. Let’s explore a few of them:
Young’s Double-Slit Experiment: In this experiment, Thomas Young demonstrated that light passing through two closely spaced slits creates an interference pattern on a screen. This pattern can only be explained by considering light as a wave.
Photoelectric Effect: Although the photoelectric effect is often associated with the particle nature of light, it also provides insights into its wave nature. The intensity of light affects the number of electrons emitted, which can be explained by the wave nature of light interacting with the electrons in a material.
Compton Scattering: Compton scattering involves the interaction of X-rays or gamma rays with electrons. The scattered radiation exhibits a shift in wavelength, which can be explained by treating light as a wave.
De Broglie Wavelength and Wave-Particle Duality
In 1924, Louis de Broglie proposed that particles, including photons, also exhibit wave-like behavior. He suggested that every particle has an associated wavelength, known as the de Broglie wavelength. This concept further deepened our understanding of the dual nature of light, which can behave both as a wave and a particle.
The de Broglie wavelength ((lambda)) of a particle is given by the equation:
![]()
where (h) is Planck’s constant and (p) represents the momentum of the particle.
Wavefunction and Superposition
In quantum mechanics, the wave nature of light is described by a mathematical function called the wavefunction. The wavefunction represents the probability distribution of finding a particle, such as a photon, at a particular location. It allows us to calculate the probability of different outcomes when measuring the position or momentum of a particle.
Superposition is another fundamental concept in quantum mechanics. It states that a particle can exist in multiple states simultaneously, represented by different wavefunctions. This principle explains phenomena like interference and the formation of wave packets.
In conclusion, the wave theory of light provides a comprehensive framework for understanding the behavior of light as both a wave and a particle. Through experiments and mathematical models, we have gained valuable insights into the wave nature of light, which has paved the way for advancements in various fields, including optics, quantum mechanics, and information technology.
Light as a Particle
Particle Theory of Light
Light, as we know it, exhibits a fascinating duality – it can behave both as a wave and as a particle. In this section, we will explore the particle theory of light and delve into the evidence that supports light’s particle nature.
The particle theory of light, also known as the corpuscular theory, proposes that light is composed of tiny discrete particles called photons. These photons carry energy and travel through space in a straight line. According to this theory, light behaves like a stream of particles, each carrying a specific amount of energy.
One of the key concepts in understanding the particle nature of light is the wave-particle duality. This principle, derived from quantum mechanics, states that particles such as photons can exhibit both wave-like and particle-like properties, depending on the experimental setup and observation.
Evidence of Light’s Particle Nature
Several experiments and phenomena provide evidence for the particle nature of light. Let’s explore some of them:
Photoelectric Effect: The photoelectric effect, first explained by Albert Einstein, demonstrates that light can transfer its energy to electrons in a material, causing them to be emitted. This phenomenon can only be explained if light is considered to consist of particles (photons) with discrete energy levels.
Compton Scattering: Compton scattering is the phenomenon where X-rays or gamma rays are scattered by electrons. This scattering can only be explained if we consider light as particles interacting with electrons, transferring momentum and energy.
Young’s Double-Slit Experiment: Young’s double-slit experiment is a classic demonstration of the wave-particle duality of light. When a beam of light passes through two closely spaced slits, it creates an interference pattern on a screen. This interference pattern can only be explained if we consider light as both a wave and a particle.
De Broglie Wavelength: According to Louis de Broglie‘s hypothesis, particles, including photons, have a wavelength associated with them. This wavelength, known as the de Broglie wavelength, is inversely proportional to the momentum of the particle. The observation of diffraction and interference patterns in experiments involving light supports the idea of light having a wave nature.
In summary, the particle theory of light provides a framework to understand the behavior of light as discrete particles called photons. Various experiments and phenomena, such as the photoelectric effect, Compton scattering, and the wave-particle duality observed in Young’s double-slit experiment, provide compelling evidence for light’s particle nature. The wave-particle duality of light is a fundamental concept in quantum mechanics, highlighting the intricate and fascinating nature of electromagnetic radiation.
The Quantum Theory of Light
Introduction to Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that describes the behavior of particles at the atomic and subatomic levels. It revolutionized our understanding of the physical world by introducing the concept of wave-particle duality. This duality suggests that particles, such as light, can exhibit both wave-like and particle-like properties.
In the early 20th century, scientists were puzzled by the nature of light. On one hand, light exhibited wave-like behavior, as demonstrated by its ability to interfere and diffract. On the other hand, it also exhibited particle-like behavior, as observed in the photoelectric effect and Compton scattering. The quantum theory of light emerged as a solution to this puzzle.
How Quantum Theory Explains Light’s Dual Nature
According to quantum theory, light is composed of tiny packets of energy called photons. These photons are the fundamental particles of light and exhibit both wave-like and particle-like properties.
The wave nature of light is evident in phenomena such as interference and diffraction. When light passes through a narrow slit or encounters an obstacle, it diffracts and creates an interference pattern. This behavior can be explained by the wave nature of light, where the photons interfere with each other constructively or destructively.
On the other hand, the particle nature of light is observed in the photoelectric effect and Compton scattering. In the photoelectric effect, light incident on a metal surface causes the ejection of electrons. This phenomenon can be explained by the particle-like behavior of photons, where each photon transfers its energy to an electron, causing it to be emitted from the surface.
The dual nature of light was further confirmed by the famous Young’s double-slit experiment. This experiment demonstrated that light could exhibit both wave-like and particle-like behavior simultaneously. When light passes through two closely spaced slits, it creates an interference pattern on a screen, indicating its wave nature. However, when detectors are placed to observe which slit the photons pass through, the interference pattern disappears, suggesting the particle nature of light.
The quantum theory of light also introduced the concept of the de Broglie wavelength. According to this concept, particles, including photons, have a wavelength associated with them. The de Broglie wavelength is given by the equation:
![]()
where ( lambda ) is the de Broglie wavelength, ( h ) is Planck’s constant, and ( p ) is the momentum of the particle.
In quantum mechanics, the behavior of particles, including photons, is described by a mathematical function called the wavefunction. The wavefunction represents the probability distribution of finding a particle at a particular location. It allows us to calculate the probability of a particle being in a certain state or having a certain energy.
One of the key principles of quantum mechanics is superposition. Superposition states that a particle can exist in multiple states simultaneously. For example, a photon can be in a superposition of different energy states. This concept helps explain phenomena such as interference and the formation of wave packets.
However, the quantum theory of light also introduced the concept of uncertainty. According to the uncertainty principle, there is a limit to how precisely we can know certain properties of a particle, such as its position and momentum, simultaneously. This principle places a fundamental limit on our ability to predict the behavior of particles.
In summary, the quantum theory of light provides a comprehensive framework for understanding the dual nature of light. It combines the wave-like and particle-like properties of light into a unified theory that explains phenomena such as interference, diffraction, the photoelectric effect, and Compton scattering. By introducing concepts such as the de Broglie wavelength, wavefunctions, superposition, and the uncertainty principle, quantum mechanics has revolutionized our understanding of light and its behavior at the microscopic level.
Implications of Light’s Dual Nature
Applications in Modern Technology
The wave-particle duality of light, which is a fundamental concept in quantum mechanics, has profound implications in various fields of modern technology. Understanding the dual nature of light has paved the way for numerous applications that have revolutionized our lives.
One of the most significant applications of light’s dual nature is in the field of telecommunications. The ability of light to behave both as a wave and a particle allows for the transmission of information through optical fibers. These fibers use the wave nature of light to carry signals over long distances with minimal loss. At the same time, the particle nature of light, specifically the photon, enables the encoding and decoding of information in the form of binary data.
Another important application of light’s dual nature is in the field of imaging and photography. The wave nature of light allows for the formation of interference and diffraction patterns, which are essential in techniques such as holography. On the other hand, the particle nature of light is utilized in digital cameras and image sensors, where photons are detected and converted into electrical signals to capture images.
Light’s dual nature also plays a crucial role in the field of spectroscopy. Spectroscopic techniques rely on the interaction of light with matter to analyze its composition and properties. The wave nature of light is utilized to study the energy quantization of atoms and molecules, while the particle nature of light is essential in techniques such as the photoelectric effect and Compton scattering, which provide valuable insights into the behavior of particles at the atomic and subatomic levels.
Impact on Scientific Understanding
The discovery of light’s dual nature has had a profound impact on our scientific understanding of the universe. It challenged the classical view of light solely as a wave and led to the development of quantum mechanics, a revolutionary theory that describes the behavior of particles at the microscopic level.
The wave-particle duality of light was first experimentally demonstrated by Thomas Young in his famous double-slit experiment. This experiment showed that light could exhibit both wave-like interference patterns and particle-like behavior, depending on the experimental setup. This groundbreaking observation paved the way for further investigations into the nature of light and the development of quantum theory.
The concept of the de Broglie wavelength, proposed by Louis de Broglie, further solidified the wave-particle duality of light. According to this concept, particles, including photons, exhibit wave-like properties with a wavelength inversely proportional to their momentum. This idea was later extended to all matter particles, leading to the development of wave mechanics and the wavefunction formalism.
The understanding of light’s dual nature and the principles of quantum mechanics have also given rise to the concept of superposition and probability distribution. These concepts allow us to describe the behavior of particles and waves in terms of wave packets and probability densities. They have become fundamental tools in modern physics, enabling us to make predictions about the behavior of particles and the outcome of experiments.
In conclusion, the implications of light’s dual nature are far-reaching and have revolutionized various fields of modern technology. From telecommunications to imaging and spectroscopy, the wave-particle duality of light has opened up new possibilities and applications. Moreover, the understanding of light’s dual nature has fundamentally changed our scientific understanding of the universe, leading to the development of quantum mechanics and its associated concepts.
Conclusion
In conclusion, light exhibits both wave and particle characteristics due to its dual nature. This phenomenon, known as wave-particle duality, was first proposed by scientists in the early 20th century. Through various experiments, it was discovered that light behaves as both a wave and a particle depending on the context. The wave nature of light explains phenomena such as interference and diffraction, while the particle nature is evident in the photoelectric effect and the emission of discrete packets of energy called photons. This duality is a fundamental aspect of quantum mechanics and has revolutionized our understanding of the nature of light.
Frequently Asked Questions
1. Why does light have wave and particle properties?
Answer: Light exhibits wave-particle duality, meaning it can behave as both a wave and a particle. This phenomenon is a fundamental aspect of quantum mechanics, where particles like photons can exhibit wave-like properties such as interference and diffraction, as well as particle-like properties such as energy quantization and the photoelectric effect.
2. What is electromagnetic radiation?
Answer: Electromagnetic radiation refers to the energy propagated through space in the form of electromagnetic waves. It includes a wide range of wavelengths, from radio waves to gamma rays, and encompasses various forms of energy, including visible light.
3. What is the wave-particle duality?
Answer: Wave-particle duality is the concept that particles, such as photons, electrons, and other subatomic particles, can exhibit both wave-like and particle-like properties. This duality is a fundamental principle of quantum mechanics.
4. What is the photoelectric effect?
Answer: The photoelectric effect is the phenomenon where electrons are emitted from a material when it is exposed to light or other forms of electromagnetic radiation. It played a crucial role in establishing the particle nature of light and led to the development of quantum mechanics.
5. What is the Compton scattering?
Answer: Compton scattering is a phenomenon where X-ray or gamma-ray photons collide with electrons, resulting in a change in the wavelength and direction of the scattered photons. This effect provided experimental evidence for the particle nature of light and confirmed the existence of photons.
6. What is the Young’s double-slit experiment?
Answer: Young’s double-slit experiment is a classic experiment that demonstrates the wave nature of light. It involves shining light through two closely spaced slits, which creates an interference pattern on a screen behind the slits, indicating the wave-like behavior of light.
7. What is the de Broglie wavelength?
Answer: The de Broglie wavelength is the wavelength associated with a moving particle, such as an electron or a photon. It is given by the ratio of Planck’s constant to the momentum of the particle and is a fundamental concept in quantum mechanics.
8. What is the uncertainty principle?
Answer: The uncertainty principle, formulated by Werner Heisenberg, states that certain pairs of physical properties, such as position and momentum, cannot be precisely known simultaneously. It implies that there are inherent limitations to our ability to measure and predict the behavior of particles at the quantum level.
9. What is a wavefunction?
Answer: In quantum mechanics, a wavefunction is a mathematical function that describes the quantum state of a particle or a system of particles. It contains information about the probabilities of different outcomes when measurements are made on the system.
10. What is a wave packet?
Answer: A wave packet refers to a localized group or bundle of waves that represents a particle in quantum mechanics. It is a superposition of different wave frequencies and is used to describe the spatial and temporal behavior of particles with wave-like properties.
Also Read:
- How to find frequency of transverse wave
- How to find energy in a cosmic microwave background experiment
- How to find amplitude of transverse wave
- Effect of wavelength on refraction
- How to calculate energy of acoustic waves
- Are transverse waves visible
- Is light a transverse wave
- Can sound waves be reflected
- How did the concept of wave particle duality come about
- How to find wavelength of transverse wave

The TechieScience Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the TechieScience.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.
